
daOur Services › CLIN-LINK file storage
Does it feel unsafe to store and share clinical files?
It doesn't have to. Our system CLIN-LINK offers safe storage and sharing of data files, videos and documents for research professionals.
With CLIN-LINK, you no longer have to worry about audits, GCP, or GDPR. Your data is safe.
It's as easy and simple as e-mailing .zip-files - but safe
It's GDPR and GCP compliant clinical file storage
Includes cloud with full privacy for your selected members (both colleagues and external partners)
CLIN-LINK supports every step of your data journey. Our clients use it for:
• Collection and storage of lab results files
• eTMF (maintenance and QC, using DIA reference model)
• Distribution of large files with sensitive information to external partners
• Collection, storage, and sharing of clinical data files (data, videos, and documents)
• Distribution of data packages to DSMB members (blinded and unblinded access areas)
• Internal QA administration (SOP, vendor and audit documentation as well as GxP systems plans)
CLIN-LINK comes in two versions
-
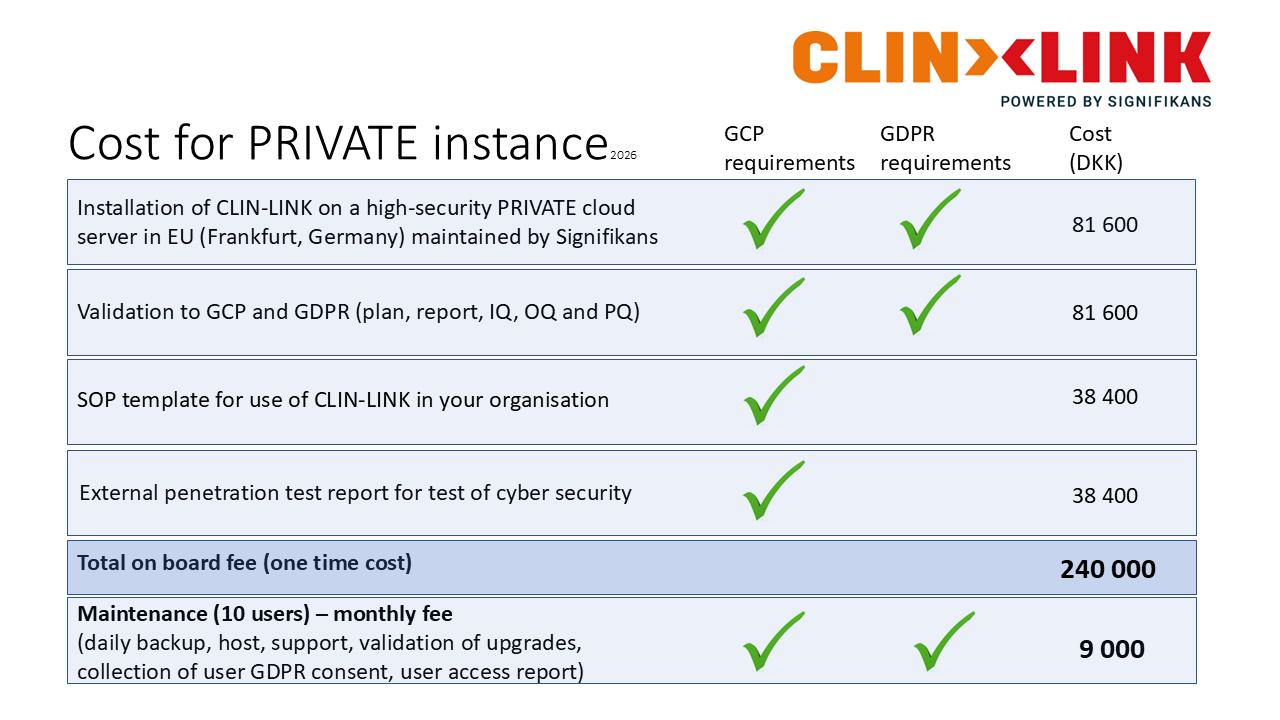
1. PRIVATE instance
Best for company use
-
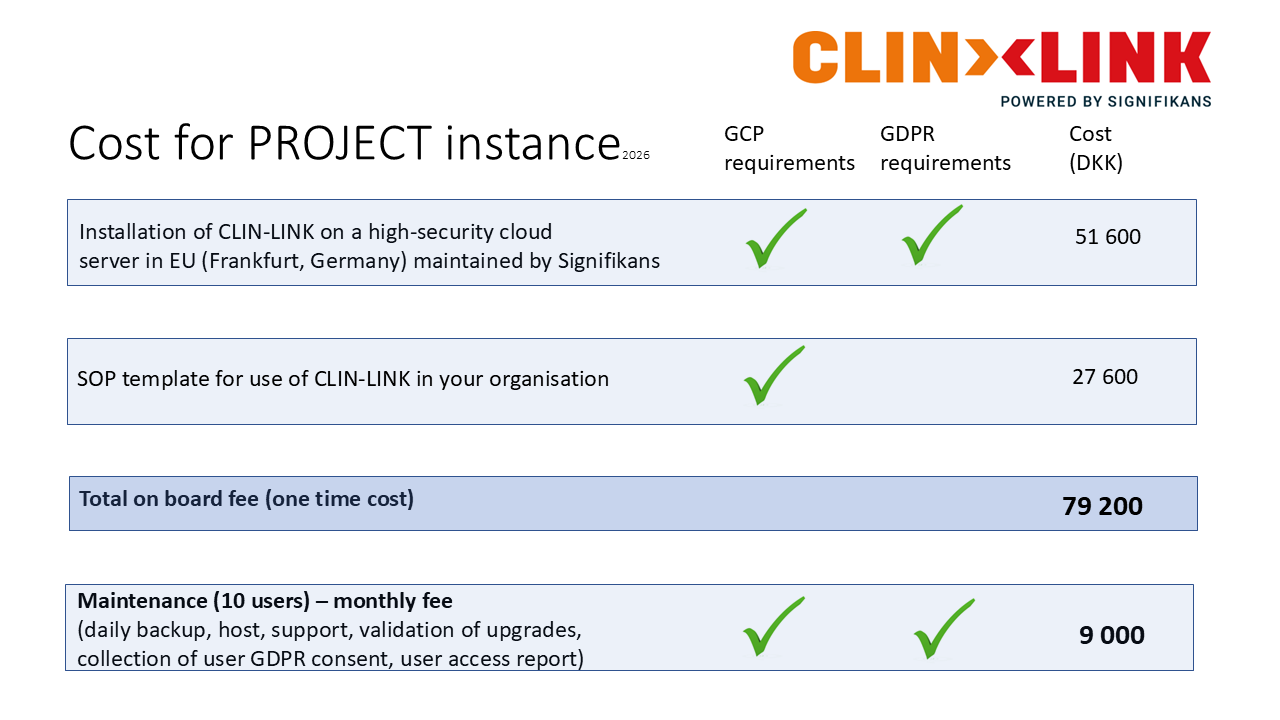
2. PROJECT instance
Best for project use
See how easy it is
Try CLIN-LINK now
Use “democlinlink” as username and password.
Explore the platform’s functionality
Watch a detailed presentation

Add-ins are available:
eTMF (storing files from your clinical trial including email to eTMF)
CLIN-DASH (visualization of your clinical and laboratory data)
Check-sum (assuring your file transfer is performed correctly)


